| Posted: April 21, 2008 | |
| (Nanowerk Spotlight) Safe, efficient and compact hydrogen storage is a major challenge in order to realize hydrogen powered transport. According to the U.S. Department of Energy's Freedom CAR program roadmap, the on-board hydrogen storage system should provide a gravimetric density of 6 wt% at room temperature to be considered for technological implementation. Currently, the storage of hydrogen in the absorbed form is considered as the most appropriate way to solve this problem. Research groups worldwide are seeking and experimenting with materials capable of absorbing and releasing large quantities of hydrogen easily, reliably, and safely. One candidate material that is being considered as a candidate for hydrogen storage media is single-walled carbon nanotubes (SWCNT). | |
| So far, carbon nanotubes have been unable to meet the DOE's hydrogen storage target. This even has led to a decision by the DOE to discontinue future applied research and development investment in pure, undoped SWCNTs for vehicular hydrogen storage applications. Although most of the previous studies have focused on hydrogen storage through physisorption, recent Density Functional Theory calculations for SWCNT indicate the potential for up to 7.5 wt% hydrogen storage capacity for this material through chemisorption (see our Spotlight: "New carbon nanotube hydrogen storage results surpass Freedom Car requirements"). | |
| New theoretical work from China suggests that silicon nanotubes can store hydrogen more efficiently than their carbon nanotube counterparts. This raises the possibility that, after powering the micro-electronics revolution, silicon could also become a key material for the future hydrogen economy. | |
| "Compared to carbon, silicon has more electrons in the outer shells, which leads to higher polarizability and a stronger dispersion force" Dr. Dapeng Cao explains to Nanowerk. "Motivated by this observation, we employ a multiscale theoretical method, which combines the first-principle calculation and a grand canonical Monte Carlo simulation, to predict the adsorption capacity of hydrogen in silicon nanotube (SiNT) arrays at a temperature of 298°K (25°C) and pressure range from 1 to 10 MPa. Our calculations show that silicon nanotubes can adsorb hydrogen molecules more efficiently than carbon nanotubes under normal fuel cell operating conditions." | |
| Cao, is a professor and vice director of the Lab of Molecular and Materials Simulation at the Beijing University of Chemical Technology. Together with other members of the Lab he published their recent findings in the March 19, 2008 online edition of The Journal of Physical Chemistry (Silicon Nanotube as a Promising Candidate for Hydrogen Storage: From the First Principle Calculations to Grand Canonical Monte Carlo Simulations). | |
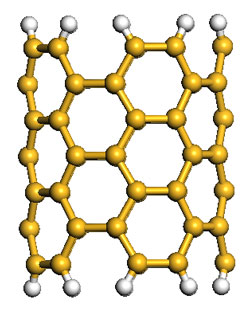 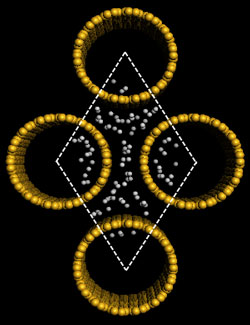 | |
| Left: Schematic representations of a (5,5) SiNT cluster model, where all the terminals are saturated with H atoms and the brown yellow and gray spheres represent Si and H atoms, respectively. Right: Gravimetric adsorption capacity of hydrogen in the rhombic SiNT array at T=298°K and P=10 MPa, resulting in 2.88 wt%. A comparative simulation with CNTs results in gravimetric density of 1.96 wt%. (Images: Dr. Cao) | |
| Following the successful synthesis of silicon nanotubes by the chemical vapor deposition method in 2002, researchers developed numerous other methods to fabricate these SiNTs and well-aligned SiNT arrays. Because silicon has more electrons in the outer shells than carbon – which leads to higher polarizability and a stronger dispersion force – scientists theorized that SiNTs may exhibit a stronger van der Waals attraction to hydrogen than CNTs. | |
| "Our multiscale theoretical method combines the first-principle calculations to obtain the binding energy between hydrogen and the SiNT and a grand canonical Monte Carlo simulation to evaluate the hydrogen adsorption capacity in the SiNT arrays, where the calculated binding energy is provided as an input in the Monte Carlo simulation" Cao explains. | |
| The researchers found that geometrical arrangement of the tubes as well as the diameter and curvature of the tube affect the adsorption of hydrogen in the SiNT array. | |
| Since SiNTs are a novel material, there is no experimental data available yet with regard to their hydrogen storage capability. Experimental work needs to be conducted to confirm this theoretical findings. Cao points out that the separation between silicon nanotubes affects the capacity of hydrogen storage significantly. Therefore one of the challenges for conducting practical experiments will be control of the optimal separation between SiNTs in the preparation of well-aligned arrays. | |
| By Michael Berger. Copyright 2008 Nanowerk LLC Source |
Monday, April 21, 2008
Silicon nanotubes could exceed their carbon counterparts in hydrogen storage efficiency
Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment