(Nanowerk Spotlight) As far as test tubes go, it doesn't get any smaller than a single-walled carbon nanotube (SWCNT). Among the wide range of interesting properties exhibited by SWCNTs is their capacity to encapsulate molecules within their quasi one-dimensional cavity. The confinement offered by the nanotube could serve as a nanoscale test tube to constrain a chemical reaction. This was demonstrated in principle back in 1998, when the coalescence of adjacent fullerenes was observed by transmission electron microscopy ("Encapsulated C60 in carbon nanotubes"). In the following years, scientists have extensively experimented with filling nanotubes with other fullerenes, atoms, molecules and, very recently, with organic molecules. Owing to their large variety with diverse chemical properties, the incorporated organic molecules can tune the properties of the SWCNTs.
Scientists are intrigued by the possibilities that SWCNTs' use as a reaction tube offers for chemistry at the nanoscale. Nanochemistry – a key to control self-assembly processes prerequisite for nanotechnology – in essence would produce stable chemical reactions inside a confined nanoscale space (it doesn't have to be a nanotube). Encapsulated inside this nanoscale space, molecules are isolated from the outside environment, which allows one to identify and control the source and incidence of chemical reactions. Recent work has demonstrated this new chemistry by using SWCNTs as a nanometer-scale reaction furnace.
"It has been known that single-walled carbon nanotubes encapsulating buckyball fullerenes can be transformed into double walled carbon nanotubes" Dr. Hidetsugu Shiozawa tells Nanowerk. "In this case, the coalescence of adjacent fullerenes triggered by Stone-Wales transformations is the accepted mechanism for the inner shell tube growth. In our recent work we demonstrated that encapsulated ferrocene molecules can also be a precursor to grow inner shell tubes. Our finding is that the encapsulated ferrocene acts in a multi role as a carbon source and a catalyst, lowering the reaction temperature of the nanotube growth down to 600°C, and as a tracking agent for the chemical reaction within the tubes."
Shiozawa, a researcher at the Advanced Technology Institute at the University of Surrey in the UK, continues by describing that, from a detailed microscopic and spectroscopic study, he and his collaborators unveiled – for the first time – that the decomposing ferrocene molecules form iron carbide nanocrystals which dissolve carbon atoms from one side and generate a carbon nanotube to the other side.
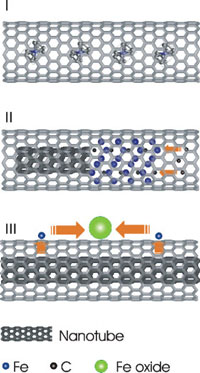
Schematic diagram for the inner tube growth process.
I) FeCp2-filled SWCNTs.
II) Catalytic reaction for the inner-tube growth after decomposition of FeCp2. The iron carbide nanocrystal acting as a reactor absorbing carbon atoms from one side and generating a nanotube to another side.
III) Precipitation of iron atoms onto the DWCNT surface and subsequent oxidation and aggregation into a nanoparticle. (Reprinted with permission from Wiley-VCH Verlag)
"Such details of chemical reactions are usually hindered by a massive reaction volume required in ordinary chemistry" he says. "It means our work elucidates the feasibility and merit of nanochemistry."
Shiozawa is first author of a paper published online on March 28, 2008 in Advanced Materials ("A Catalytic Reaction Inside a Single-Walled Carbon Nanotube") that is the result of an international collaboration of scientists from the Leibniz Institute for Solid State and Materials Research (IFW) Dresden in Germany, the University of Vienna in Austria, and the National Institute of Advanced Industrial Science and Technology (AIST) in Japan.
The research team found that filling a carbon nanotube leads to electron doping of the tube. Furthermore, in separate work, Shiozawa has demonstrated that the chemical reaction of filled ferrocene induces a dynamic electron or hole doping of the outer tube (these findings have been published online on April 2, 2008 in Physical Review B – " Fine tuning the charge transfer in carbon nanotubes via the interconversion of encapsulated molecules").
These results illustrate the applicability of this confined chemical reaction in functionalizing the electronic properties of carbon nanotubes. By choosing a proper type of filling materials, switching the electronic and optical properties of carbon nanotubes may be possible.
Shiozawa also notes that the catalytic process at the nanoscale leads to the growth of iron oxide nanoparticles on the carbon nanotubes. "Expected diverse magnetic properties of such nanoparticles may render a range of new biomedical and diagnostic applications" he says. "Since carbon nanotubes represent a new class of molecular transporters potentially useful for future drug delivery, our ultimate product, i.e., iron oxide nanoparticles supported by carbon nanotubes, can be an ideal composite for proving the concepts of biomedical functionality and activity of such nanosystem."
With the demonstration that nanochemistry is possible within the inner space of carbon nanotubes, this new approach can potentially be applied to all kinds of chemistry by choosing reasonable combinations of guest materials and host nanostructures. Shiozawa believes this will trigger a transition of ordinary chemistry into nanochemistry where the designed nanospace induces novel chemical reactions.
"Given a large variety of organometallic precursors with transition metal and rare-earth metal atoms, our process will advance the development of a diverse assembly of atoms, molecules, nanocrystals and carbon nanotubes. Such nanocomposites will provide a model base for exploring exotic quantum physical phenomena, which advances our understanding of solid-state physics."
By Michael Berger. Copyright 2008 Nanowerk LLC
Source
No comments:
Post a Comment