September 18, 2008
WEST LAFAYETTE, Ind. - How fat cells become after being exposed to a specialized electrical field is helping researchers determine whether cells are normal, cancerous or a stage of cancer already invading other parts of the body.
Purdue University scientists tested the electrical process and found cells that expanded the most were metastatic cancer, the term used when the disease has spread beyond its point of origin. The technique allows screening of single cells 300 times faster - five cells per second compared with the one cell per minute of previous methods, said Chang Lu, senior and corresponding author of the study currently online in the journal Analytic Chemistry. This rapid cell inspection permits testing of enough cells for diagnosis and determination of the disease's level, he said.
"If you look at the properties of only a few cells, it would be a stretch to say they exactly represent a tissue cell population since tissues have tens of thousands of cells," Lu said. "Our goal is to have a tool so that we can reputably look at large numbers of cells and obtain information about their biomechanical properties."
Using breast cancer cells, Lu and his research team investigated cancer cells at different stages and compared their size to normal cells after all three types of cells were treated with the electrical process.
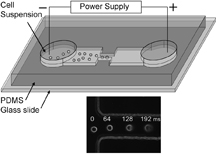 |
The cell expansion results because as cancer develops, it compromises the cell's structure, or cytoskeleton. A metastatic cancer cell cytoskeleton is more prone to deformity than a primary cancer cell or a normal cell, said Lu, an assistant professor of agricultural and biological engineering. When cells were put through the electrical field that Lu and his colleagues used, pores opened in the cell membrane, allowing fluid from the microchannel into the cell itself.
Just like a person gaining a lot of weight, the cells balloon in size, with the biggest deformity appearing in the metastatic cells.
"Our approach - microfluidic electroporative flow cytometry - can exactly characterize the degree to which a cell can become deformed," Lu said.
A patent is pending on the technique.
The amount of electricity and the length of time the cell is exposed to it also determined how much the various types of cells expanded. Under the parameters that Lu's team used, the metastatic cells expanded by 75 percent after electroporation, while primary cancer cells and normal cells expanded by 50 percent and 25 percent, respectively.
The Purdue scientists used a camera to measure how many cells were being screened over a predetermined time period. The camera must be able to capture changes in each cell one frame at a time.
The scientists want to make the screening process even faster, so they need a camera that can shoot frames even more rapidly than the one Lu's team used for this study. This would provide more accurate measurement of cell expansion. They also want to use the technique to diagnose other types of cancers and other diseases. Tests on a blood disease already are under way.
The eventual goal is to apply microfluidic electroporative flow cytometry in patient trials, Lu said.
"But things become more complicated if you're dealing with patient tissues rather than cell lines," he said. "Cell lines contain only one type of cell; patient tissues have different kinds of cells."
The Wallace H. Coulter Foundation and the National Science Foundation funded this study.
Lu also has appointments in Purdue's Weldon School of Biomedical Engineering, School of Chemical Engineering and the Laboratory for Renewable Resources Engineering. Department of Agricultural and Biological Engineering postdoctoral student Ning Bao and graduate student Yihong Zhan co-authored the paper.
Writer: Susan A. Steeves, (765) 496-7481, ssteeves@purdue.edu
Source: Chang Lu, (765) 494-1188, changlu@purdue.edu
Ag Communications: (765) 494-2722;
Beth Forbes, forbes@purdue.edu
Agriculture News Page
IMAGE CAPTION:
This schematic shows a microfluidic electroporative flow cytometer. The inset image shows the continuous increase in the cell size as the cell flows in the electrical field. (Purdue University graphic)
Microfluidic Electroporative Flow Cytometry for Studying Single Cell Biomechanics
Biomechanical properties of cells yield important information on the disease state of cells, such as transformation and metastasis. Screening of cells based on their biomechanical properties provides rapid tools for label-free diagnosis and staging of cancers. However, existent single cell techniques for measuring biomechanical properties suffer from low throughput (min). This prevents the application of these assays to a large cell population, which produces information with statistical significance. In this study, we applied microfluidics-based electroporative flow cytometry (EFC) that combined electroporation with flow cytometry to study deformability of cells at the single cell level with a throughput of ~5 cells/s. The cell swelling during flow-through electroporation was recorded in real time. We believe that the degree of such swelling was indicative of the cell deformability and the cytoskeleton mechanics. Three cell types (MCF-10A, MCF-7 and 12-O-tetradecanoylphorbol-13-acetate (TPA) treated MCF-7) with different malignancy and metastatic potential were tested using our approach. We found that the more malignant and metastatic cell types exhibited more swelling due to higher cell deformability. Furthermore, the disruption of microtubules by colchicine caused substantial change in the EFC results, which confirmed that EFC data strongly reflected the cytoskeletal mechanics. Finally, the cell type with the highest metastatic potential also suffered the most cell death due to the flow-through electroporation treatment, presumably due to the most substantial cell swelling which could irreversibly rupture the membrane. EFC provides a new method for examining single cell biomechanics with high throughput. We believe that this technique will be useful for mechanistic studies of cytoskeleton dynamics and clinical applications such as diagnosis and staging of cancers in general.
Ref:
National Science Foundation awards prestigious grant to Purdue researcher
Publication Date: 11/14/2008
Web Release Date: September 18,
Microfluidic Electroporative Flow Cytometry for Studying Single-Cell Biomechanics
Department of Agricultural and Biological Engineering, Weldon School of Biomedical Engineering, School of Chemical Engineering, Purdue University, West Lafayette, Indiana 47907