This is a review article that summarized the research projects undertaken in my lab. It also introduced several related activities from among other groups. Several of our projects have been of interest to public readers. This paper contains much new information and I think this may have been why it has been highly cited. It describes new concepts and methodologies, particularly the concept of nanoparticle self-lighting photodynamic therapy for cancer treatment, the concept of combining photodynamic therapy and radiation therapy for deep cancer treatment, and the applications of nanoparticle photosensitizers for cancer treatment. These are brand new approaches in nanotechnology. In this paper, we introduced several new developments in nanotechnology for biological applications. For example, nanoparticle-based photodynamic therapy is one of the most exciting approaches described in this article. The goal is to find an efficient cancer therapy by developing X-ray luminescence nanoparticles as a light source for photodynamic therapy. Photodynamic therapy has been designated as a "promising new modality in the treatment of cancer" since the early 1980s. Light must be delivered in order to activate photodynamic therapy. Most drugs used for photodynamic therapy require ultraviolet or blue light for activation. Unfortunately, ultraviolet and blue light have minimal penetration into tissue and their application for deep cancer therapy is a problem.
To solve this problem and also to enhance the treatment for deep cancers, we proposed a new photodynamic therapy system in which the light is generated by X-ray luminescence nanoparticles. The X-ray luminescence nanoparticles and afterglow nanoparticles are attached to photoactive drugs and, when the nanoparticle-drug systems are targeted to the tumor and are stimulated by an X-ray during radiotherapy, these nanoparticles will generate light (energy) which activate the drugs for photodynamic therapy. In this case, no direct light delivery to the tumor is necessary and very low doses of radiation are needed. In this modality, the radiation and photodynamic therapies are combined and occur simultaneously, so that the tumor destruction will be more efficient. More importantly, it can be used for deep tumor treatment, as X-rays can penetrate deeply into the tissue. Once demonstrated, this will provide a simple but more efficient modality for breast cancer treatment. I have been working on nanotechnologies for the past 15 years. My original work concentrated on trying to use quantum dots for in vivo imaging, with a concentration on the challenge of light penetration. I also have experience with the design and synthesis scintillation measurement for nanoparticles. I knew light delivery was also a challenging issue for photodynamic therapy, just like in vivo optical imaging. I then arrived at the idea to combine photodynamic therapy with radiation therapy through scintillation nanoparticles for deep cancer treatment. Photodynamic therapy is not new, nor is radiation therapy, but the combination of both through scintillation nanoparticles is new and potentially important for deep cancer treatment. I introduced the concept in a paper published in the Journal of Nanoscience and Nanotechnology in 2006. (Wei Chen and Jun Zhang, "Using Nanoparticles to Enable Simultaneous Radiation and Photodynamic Therapies for Cancer Treatment," Journal of Nanoscience and Nanotechnology 6[4]: 1159-66, April, 2006). Initial results of the studies have been promising. But before "nanoparticle self-lighting photodynamic therapy" becomes a clinical reality, researchers must overcome two main challenges: 1) they need to develop a class of water-soluble scintillation nanoparticles with very high quantum efficiencies of X-ray luminescence, and 2) they need to improve the targeting capabilities of the nanoparticle-photosensitizer compound—but this is a challenge for all drug-based cancer treatments. Most recently, we've used afterglow nanoparticles for photodynamic therapy activation. This is a good solution for improving efficiency since afterglow nanoparticles will maintain their luminescence for a certain period of time after activation. In this case, the radiation dose will be reduced exponentially. This new concept is also introduced in a recent publication: Chen, W. "Nanoparticle self-Lighting photodynamic therapy for cancer treatment," J. Biomed. Nanotechnol 4[4]: 369-76, 2008. This concept is getting more popular and has become intriguing to many investigators. I think this will become quite a hot area during the next decade and that products for practical applications will, sooner or later, be realized. One of our recent review papers has appeared on the ScienceDirect "25 Hottest Articles" list for October—December, 2008: (Juzenas, P., et al.,"Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer," Advanced Drug Delivery Reviews [60]15: 1600-14, December 2008). By the way, many cancer patients and/or their families have contacted me directly and enquired as to the timing for this new modality to become available for patient treatment. I have sensed this urgent need and that is the key motivation for my research. Although there are no obvious political implications for my research, the slowing economy in the USA has really affected the funding of many new scientific projects. Wei Chen, Ph.D. | |||||||
Sunday, May 31, 2009
Combining photodynamic therapy and radiation therapy for deep cancer treatment,
Monday, February 11, 2008
Nanoparticle self-lighting photodynamic therapy for deep cancer treatment
| Posted: February 11, 2008 | |
| Nanoparticle self-lighting photodynamic therapy for deep cancer treatment | |
| (Nanowerk Spotlight) Photodynamic therapy (PDT) is a cancer treatment that combines a chemical compound, called a photosensitizer, with a particular type of light to kill cancer cells. The treatment works like this: the photosensitizing agent is injected into the bloodstream. The agent is absorbed by cells all over the body, but stays in cancer cells longer than it does in normal cells. One to three days after injection, when most of the agent has left normal cells but remains in cancer cells, the tumor is exposed to light. The photosensitizer in the tumor absorbs the light and produces an active form of oxygen (singlet oxygen) that destroys nearby cancer cells. PDT has been used for the past 30 years and is a treatment that works. PDT takes very little time, is often done as an outpatient, can be accurately targeted to the affected area, can be repeated, and has no long-term side effects. It also isn't as expensive or invasive as some other cancer treatment options. The limitation of this form of cancer treatment is that the light needed to activate most photosensitizers cannot pass through more than one centimeter of tissue. For this reason, PDT is usually used to treat tumors on or just under the skin or on the lining of internal organs or cavities. PDT is also less effective in treating large or deep tumors, because the light cannot pass far into these tumors. Researchers have now proposed a new PDT system in which the light is generated by x-ray scintillation nanoparticles with attached photosensitizers. When the nanoparticle-photosensitizer conjugates are targeted to tumors and stimulated by x-rays during radiotherapy, the particles generate visible light that can activate the photosensitizers for photodynamic therapy. Therefore, the radiation and photodynamic therapies are combined and occur simultaneously, and the tumor destruction can be more efficient. More importantly, it can be used for deep tumor treatment as x-rays can penetrate through tissue. | |
| "I have been working on nanotechnologies for 15 years" Dr. Wei Chen tells Nanowerk. "My original work was trying to use quantum dots for in vivo imaging. I was facing the challenge of light penetration. I also have experience with the design and synthesis scintillation nanoparticles. I knew light delivery was also a challenging issue for PDT, just like in vivo optical imaging. Then, I came up with the idea to combine photodynamic therapy with radiation therapy through scintillation nanoparticles for deep cancer treatment." | |
| Chen, an assistant professor of Nano-Bio Physics at the University of Texas at Arlington, points out that photodynamic therapy is not new, and radiation therapy is not new; but the combination of both through scintillation nanoparticles is new and potentially important for deep cancer treatment. He introduced the concept in a paper in the Journal of Nanoscience and Nanotechnology in April 2006 ("Using Nanoparticles to Enable Simultaneous Radiation and Photodynamic Therapies for Cancer Treatment"). | |
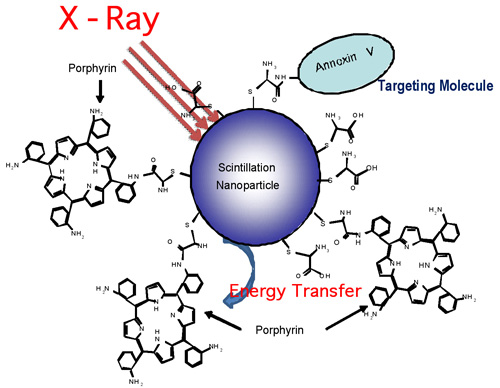 | |
| A schematic illustration of nanoparticle–porphyrin conjugates for X-ray stimulated photodynamic therapy for cancer treatment. Annexin V is a molecule that can target some specific antigens at tumor cells. (Image: Dr. Chen, University of Texas at Arlington) | |
| Although PDT has been widely used for skin cancer treatment, its application for deep cancer treatment is still a challenging issue because the light for PDT activation cannot penetrate deep into the tissue. To solve this problem, Chen and his collaborators propose a new PDT system in which the light is generated by scintillation luminescence nanoparticles (such as X-ray luminescence nanoparticles) with the attached photosensitizers. | |
| Chen explains that, when the nanoparticle-photosensitizer conjugates are targeted to a tumor and stimulated by X-ray or other radiation sources during radiation therapy, the particles will generate light (energy) to activate the photosensitizers. With this novel therapeutic approach, no external light is necessary to activate the photosensitizing agent within tumors. Tissue thickness therefore would no longer be a limiting issue for PDT. | |
| "Effectively, the radiation and photodynamic therapies are combined and occur simultaneously, and the tumor destruction will be more effective" he says. "More importantly, it can be used for deep tumor treatment as X-ray can penetrate deep into the tissue. No external light is necessary to deliver to the tumor and only an extremely low dose of radiation is needed for the treatment. Therefore, this provides a simple but more efficient modality for cancer treatment. We called this new modality Nanoparticle Self-Lighting Photodynamic Therapy." | |
| Working with Chen's group are Dr. Shaopeng Wang and Dr. Yuanfang Liu, senior research scientists at ICx/Nomadics Inc.; Dr. Alan G. Joly, an optical physicist and a senior scientist at Pacific Northwest National Laboratory; and Dr. Carey Pope, Regents Professor And Head Sitlington Chair In Toxicology at the Center for Veterinary Health Sciences, Oklahoma State University. | |
| The researchers reported their findings in a recent paper published in the January 29, 2008 online edition of Applied Physics Letters ("Investigation of water-soluble x-ray luminescence nanoparticles for photodynamic activation"). | |
| Their pilot studies indicate that water-soluble scintillation nanoparticles (the particle size in the study was about 15 nm) can potentially be used to activate photodynamic therapy as a promising deep cancer treatment modality. | |
| For practical applications, the nanoparticle-porphyrin conjugates must be delivered to the tumor cells in vehicles such as antibodies, peptides, liposomes or other functional molecules. In designing the delivery vehicles one needs to consider how they will affect the quantum yield of singlet oxygen. Chen and his team used folic acid to target folate receptors at tumor cells. Their results indicate that folic acid has no effect on the quantum yield of singlet oxygen production in the nanoparticle conjugates, making this system practical for photodynamic activation applications. | |
| Initial results of the studies have been promising. But before Nanoparticle Self-Lighting Photodynamic Therapy becomes a clinical reality, the researchers must overcome two main challenges: 1) they need to develop a class of water-soluble scintillation nanoparticles with very high quantum efficiencies of X-ray luminescence, and 2) they need to improve the targeting capabilities of the nanoparticle- photosensitizer compound – but this is a challenge for all drug-based cancer treatments. | |
| By Michael Berger. Copyright 2008 Nanowerk LLC http://www.nanowerk.com/spotlight/spotid=4466.php |
Wednesday, March 18, 2009
Nanoparticle self-lighting photodynamic therapy for deep cancer treatment
UpdateTime: 2008-9-21 20:18:33 Hits: 231 Keyword: photodynamic, cancer treatment
Posted February 13 2008
(Nanowerk Spotlight) Photodynamic therapy (PDT) is a cancer treatment that combines a chemical compound, called a photosensitizer, with a particular type of light to kill cancer cells. The treatment works like this: the photosensitizing agent is injected into the bloodstream. The agent is absorbed by cells all over the body, but stays in cancer cells longer than it does in normal cells. One to three days after injection, when most of the agent has left normal cells but remains in cancer cells, the tumor is exposed to light. The photosensitizer in the tumor absorbs the light and produces an active form of oxygen (singlet oxygen) that destroys nearby cancer cells. PDT has been used for the past 30 years and is a treatment that works. PDT takes very little time, is often done as an outpatient, can be accurately targeted to the affected area, can be repeated, and has no long-term side effects. It also isn't as expensive or invasive as some other cancer treatment options. The limitation of this form of cancer treatment is that the light needed to activate most photosensitizers cannot pass through more than one centimeter of tissue. For this reason, PDT is usually used to treat tumors on or just under the skin or on the lining of internal organs or cavities. PDT is also less effective in treating large or deep tumors, because the light cannot pass far into these tumors.
Researchers have now proposed a new PDT system in which the light is generated by x-ray scintillation nanoparticles with attached photosensitizers. When the nanoparticle-photosensitizer conjugates are targeted to tumors and stimulated by x-rays during radiotherapy, the particles generate visible light that can activate the photosensitizers for photodynamic therapy. Therefore, the radiation and photodynamic therapies are combined and occur simultaneously, and the tumor destruction can be more efficient. More importantly, it can be used for deep tumor treatment as x-rays can penetrate through tissue. "I have been working on nanotechnologies for 15 years" Dr. Wei Chen tells Nanowerk. "My original work was trying to use quantum dots for in vivo imaging. I was facing the challenge of light penetration. I also have experience with the design and synthesis scintillation nanoparticles. I knew light delivery was also a challenging issue for PDT, just like in vivo optical imaging. Then, I came up with the idea to combine photodynamic therapy with radiation therapy through scintillation nanoparticles for deep cancer treatment." Chen, an assistant professor of Nano-Bio Physics at the University of Texas at Arlington, points out that photodynamic therapy is not new, and radiation therapy is not new; but the combination of both through scintillation nanoparticles is new and potentially important for deep cancer treatment. He introduced the concept in a paper in the Journal of Nanoscience and Nanotechnology in April 2006 ("Using Nanoparticles to Enable Simultaneous Radiation and Photodynamic Therapies for Cancer Treatment"). Although PDT has been widely used for skin cancer treatment, its application for deep cancer treatment is still a challenging issue because the light for PDT activation cannot penetrate deep into the tissue. To solve this problem, Chen and his collaborators propose a new PDT system in which the light is generated by scintillation luminescence nanoparticles (such as X-ray luminescence nanoparticles) with the attached photosensitizers. Chen explains that, when the nanoparticle-photosensitizer conjugates are targeted to a tumor and stimulated by X-ray or other radiation sources during radiation therapy, the particles will generate light (energy) to activate the photosensitizers. With this novel therapeutic approach, no external light is necessary to activate the photosensitizing agent within tumors. Tissue thickness therefore would no longer be a limiting issue for PDT. "Effectively, the radiation and photodynamic therapies are combined and occur simultaneously, and the tumor destruction will be more effective" he says. "More importantly, it can be used for deep tumor treatment as X-ray can penetrate deep into the tissue. No external light is necessary to deliver to the tumor and only an extremely low dose of radiation is needed for the treatment. Therefore, this provides a simple but more efficient modality for cancer treatment. We called this new modality Nanoparticle Self-Lighting Photodynamic Therapy." Working with Chen's group are Dr. Shaopeng Wang and Dr. Yuanfang Liu, senior research scientists at ICx/Nomadics Inc.; Dr. Alan G. Joly, an optical physicist and a senior scientist at Pacific Northwest National Laboratory; and Dr. Carey Pope, Regents Professor And Head Sitlington Chair In Toxicology at the Center for Veterinary Health Sciences, Oklahoma State University. The researchers reported their findings in a recent paper published in the January 29, 2008 online edition of Applied Physics Letters ("Investigation of water-soluble x-ray luminescence nanoparticles for photodynamic activation"). Their pilot studies indicate that water-soluble scintillation nanoparticles (the particle size in the study was about 15 nm) can potentially be used to activate photodynamic therapy as a promising deep cancer treatment modality. For practical applications, the nanoparticle-porphyrin conjugates must be delivered to the tumor cells in vehicles such as antibodies, peptides, liposomes or other functional molecules. In designing the delivery vehicles one needs to consider how they will affect the quantum yield of singlet oxygen. Chen and his team used folic acid to target folate receptors at tumor cells. Their results indicate that folic acid has no effect on the quantum yield of singlet oxygen production in the nanoparticle conjugates, making this system practical for photodynamic activation applications. Initial results of the studies have been promising. But before Nanoparticle Self-Lighting Photodynamic Therapy becomes a clinical reality, the researchers must overcome two main challenges: 1) they need to develop a class of water-soluble scintillation nanoparticles with very high quantum efficiencies of X-ray luminescence, and 2) they need to improve the targeting capabilities of the nanoparticle- photosensitizer compound – but this is a challenge for all drug-based cancer treatments. By Michael Berger. Copyright 2008 Nanowerk LLC
Thursday, October 29, 2009
Nanoparticle Self-Lighting Photodynamic Therapy For Cancer Treatment
Nanoparticle Self-Lighting Photodynamic Therapy For Cancer Treatment
Wei Chen*
Department of Physics, University of Texas at Arlington, Arlington, TX 76019-0059
Photodynamic therapy (PDT) has been designated as a “promising new modality in the treatment of cancer” since the early 1980s. Light must be delivered in order to activate photodynamic therapy. Most photosensitizers have strong absorption in the ultraviolet (UV) – blue range, therefore, UV -blue light is needed for their activation. Unfortunately, UV-blue light has minimal penetration into tissue and its application for in vivo activation is a problem. To solve the problem and to enhance the PDT treatment for deep cancers, we introduce a new PDT system in which the light is generated by afterglow nanoparticles with attached photosensitizers. When the nanoparticle-photosensitizer conjugates are targeted to tumor, the light from afterglow nanoparticles will activate the photosensitizers for photodynamic therapy. Therefore, no external light is required for treatment. More importantly, it can be used to treat deep tumor such as breast cancer because the light source is attached to the photosensitizers and are delivered to the tumor cells all together. This new modality is refereed as Nanoparticle Self-Lighting Photodynamic Therapy (NSLPDT).
Key Words: Photodynamic Therapy, Cancer, Nanoparticles, Quantum Dots, Luminescence, Afterglow, Penetration, Radiation Therapy.
Corresponding Author: weichen@uta.edu
Other articles on Wei Chen