| (Nanowerk Spotlight) Quite a number of serious medical conditions, such as cancer, diabetes and chronic pain, require medications that cannot be taken orally, but must be dosed intermittently, on an as-needed basis, and over a long period of time. Researchers have been trying to develop drug delivery techniques with 'on-off switches' that would allow controlled release of drugs into the body. These methods use stimuli such as an implanted heat source or an implanted electronic chip to trigger the drug release from the implanted reservoir. So far, none of these methods can reliably perform all the needed actions: repeatedly turn dosing on and off, deliver consistent doses, and adjust doses according to each patient's need. | |
| By combining magnetism with nanotechnology, researchers have now created a small implantable device that encapsulates the drug in a specially engineered membrane, embedded with magnetic iron oxide nanoparticles. The application of an external, alternating magnetic field heats the magnetic nanoparticles, causing the gels in the membrane to warm and temporarily collapse. This collapse opens up pores that allow the drug to pass through and into the body. When the magnetic field is turned off, the membranes cool and the gels re-expand, closing the pores and halting drug delivery. No implanted electronics are required. | |
| "We have developed an implantable system that can provide on-demand, reproducible drug release whenever the patient – or other operator – wants, for as long as needed, and with the intensity that is desired, using a trigger that is external to the body – in this case an oscillating magnetic field," Daniel Kohane tells Nanowerk. "Most of the previously designed systems could only result in a single release event, or involved implanted triggering systems, or connectors to the outside world." | |
| Kohane, an associate professor of anesthesiology at Harvard Medical School and a senior associate in critical care medicine at Children's Hospital Boston, and his team have reported their findings in a recent issue of Nano Letters ("A Magnetically Triggered Composite Membrane for On-Demand Drug Delivery"). | |
| Kohane explains that composite membrane-based drug delivery devices have the potential to greatly increase the flexibility of pharmacotherapy and improve the quality of patients' lives by providing repeated, long-term, on-demand drug delivery for a variety of medical applications, including the treatment of pain (local or systemic anesthetic delivery), local chemotherapy, and insulin delivery. | |
| The membrane that Kohane's team developed consists of ethyl cellulose (the membrane support), superparamagnetic magnetite nanoparticles (the triggering entity), and thermosensitive poly(N-isopropylacrylamide) (PNIPAM)-based nanogels (the switching entity). Membranes were prepared by co-evaporation so that the nanogel and magnetite nanoparticles were entrapped in ethyl cellulose to form a presumably disordered network. To facilitate effective in vivo triggering, the nanogels were engineered to remain swollen (i.e., in the 'off' state) at body temperature. | |
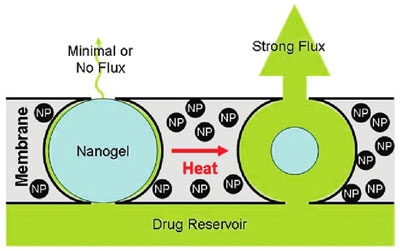 | |
| Stimulus-responsive membrane triggering in vitro: schema of the proposed mechanism of membrane function. (Reprinted with permission from American Chemical Society) | |
| "When we subjected the magnetic nanoparticles embedded in the membrane to an external oscillating magnetic field, they heated inductively," explains Kohane. "The heat generated by magnetite induction heating was transferred to the adjacent thermosensitive nanogels, causing the nanogels to shrink and permit drug diffusion out of the device. When we turned off the magnetic field, the nanogels cooled, causing them to reswell, turning off the drug flow and refilling the membrane pores." | |
| The researchers observed a 10- to 20-fold differential flux between the 'off' and 'on' states. Furthermore, multiple on-off cycles could be performed without significantly changing the permeability of the membrane in the off state. | |
| The on-off action doesn't occur immediately but was much more rapid than that seen with bulk, interpenetrating hydrogel networks. The devices turned 'on' with only a 1-2 minute time lag after the solution temperature reached 40°C and turned 'off' with a 5-10 minute lag after the stimulus was switched off. | |
| Kohane points out that reproducibility will clearly be a key consideration in devices of this type, especially with drugs with narrow therapeutic indices. | |
| "We have shown excellent reproducibility over four magnetically induced cycles" he says. "The maximum number of cycles over which that reproducibility can be maintained remains to be determined, as does the number of cycles over which it needs to be maintained. The latter will depend to a large extent on the specific clinical indication and the expected duration of therapy. Some devices might only need to be triggered a few times, while others – e.g., for a chronic condition requiring treatment several times a day – might require reproducible triggering over thousands of cycles. This issue will be of great importance in the downstream development of the device. Indeed, the ultimate design of a clinical drug delivery device based on this membrane technology, including the specific materials of which it will be composed, is yet to be determined." | |
| By Michael Berger. Copyright 2009 Nanowerk LLC | |
Monday, November 16, 2009
Remote-controlled nanocomposite for on-demand drug delivery inside the body
Monday, January 19, 2009
Swallowing a nanotechnology pill
| Posted: January 19, 2009 | |
| (Nanowerk Spotlight) Typically, nanoparticles have been used for drug delivery and it is only recently that carbon nanotubes (CNTs) have gained attention as potential drug delivery vehicles (see: "Nanotechnology's magic bullet "). Carbon nanotubes offer a number of advantages which suggest that they may provide an improved result over nanoparticles. They have a larger inner volume which allows more drug molecules to be encapsulated, and this volume is more easily accessible because the end caps can be easily removed, and they have distinct inner and outer surfaces for functionalization. Current research has shown the ability of CNTs to carry a variety of molecules such as drugs, DNA, proteins, peptides, targeting ligands etc. into cells – which makes them suitable candidates for targeted delivery applications. Despite these advantages, a suitable delivery system has not been developed yet for the targeted delivery of CNTs to specific sites. | |
| A research team from various Canadian and U.S. universities has now demonstrated, for the first time, the design and development of a novel microcapsule carbon nanotube targeted delivery device. | |
| "Our results have shown that carbon nanotubes functionalized with therapeutic molecules can be embedded into the core or at the surface of different types of alginate capsules to form novel polymeric membrane CNT microcapsules," Satya Prakash tells Nanowerk. "The membrane offers protection to the drug being carried, while the CNTs help achieve targeted delivery of the therapeutic." | |
| As CNTs can be functionalized with drugs and biomarkers specific to a disease, this device can be targeted for a specific site for optimal clinical benefits and it can easily limit the exposure of the drug to healthy tissues of the body to overcome potential drug induced toxic side-effects. | |
| Prakash, an associate professor in biomedical engineering, at McGill University and director of the university's Biomedical Technology and Cell Therapy Research Laboratory, and his collaborators expect their device to have great potential in the delivery of drugs, genes, proteins and other therapeutic molecules. Together with scientists from Rensselaer Polytechnic Institute, Southern Illinois University, and Rice University he has published the team's findings in the January 14, 2009 print edition of Nanotechnology ("Microcapsule carbon nanotube devices for therapeutic applications"). | |
| "There is abundant literature in the area of functionalizing CNTs with a variety of therapeutics" says Prakash. "However, almost all studies so far have been focusing on the systemic delivery of the CNTs. Oral delivery is the most convenient route of administration for therapeutics and also offers the advantage of achieving targeted delivery, specifically for diseases of the gastrointestinal tract. There was a need to combine the advances in carbon nanotube research with those in drug delivery systems. Our study, for the first time, investigates the feasibility of designing a polymeric membrane microcapsule CNT device that can be used in oral delivery applications." | |
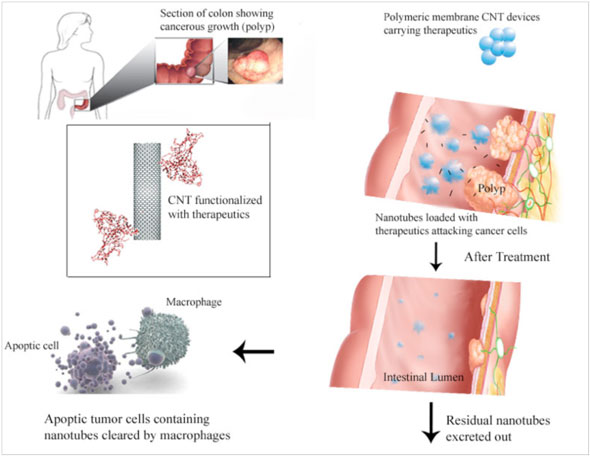 | |
| Example of a polymeric membrane CNT device in colon targeted delivery applications. (Image: Dr. Prakash, McGill University) | |
| So far, the use of CNTs in oral drug delivery – which is one of the most common and convenient route of therapeutics administrations – has not been possible to achieve. During oral delivery a drug must survive the harsh conditions of the gastrointestinal tract and retain its potency to be effective, and CNTs must be released with their cargo at a specific gastrointestinal site. | |
| The team used single-walled CNTs, which they functionalized with hydrophilic carboxylic acid and hydroxyl groups, and then embedded them in the core of alginate microcapsules or coated them on their surface. By using an automated microencapsulator they were able to obtain highly uniform capsules. | |
| "The nanotubes on the capsule surface can be functionalized with antibodies to promote adhesion of the capsules to specific target sites in vivo, thereby facilitating targeted delivery while the embedded CNTs can be functionalized with suitable biomolecules for drug/gene delivery at the site of adhesion upon degradation of the capsule at the target site," explains Prakash. "The biocompatibility of the nanotubes and the encapsulation materials, which has been already established through various studies, makes it possible to use this device for therapeutic purposes." | |
| A particular challenge for the medical application of carbon nanotubes will be their biocompatibility, something which currently is a considerable area of concern, especially with conflicting reports on their toxicity (see: "Comparing apples with oranges - the problem of nanotubes risk assessment "). Long term toxicity studies will have to be conducted in order to establish the safety of this device. | |
| By Michael Berger. Copyright 2008 Nanowerk LLC | |
Thursday, August 28, 2008
Advances in nanomedicine - understanding the intricacies of nanoparticle drug delivery
| Posted: August 28, 2008 | |
| (Nanowerk Spotlight) Nanomedicine, especially drug delivery with nano-sized drug carriers, is all the rage these days. The concept sounds simple: make nanoscale containers that can escape detection by the body's defense mechanisms, fill them with a drug, get them to the desired location within the body, release the drug payload and, presto, you've got a very effective and efficient weapon for instance to fight cancer. That this model works in principle has already been demonstrated in numerous studies. The same studies show the complicated nature and the many difficulties that scientists are facing in fabricating the right nanocontainers, getting them to the right location, controlling the release mechanism of the drug, measuring the drugs' efficacy, and monitoring the now empty delivery vehicles' fate. | |
| In a previous Spotlight – Mathematical engines of nanomedicine – we described the vast complexities in designing effective nanoparticles that take into account a wide range of possible design parameters (such as size, shape, surface properties, bulk properties, surface density of targeting moieties) and the biological characteristics of the cellular target in the body (such as receptor density, blood-flow descriptors, wall permeability). The findings we described in this article also indicate that almost all the nanocarriers that are in the clinic or in the preclinical pipeline today are basically the worst possible size and shape for their intended purpose. | |
| That engineered nanomaterials, especially inorganic ones, will be used for nanomedicine applications has now become a certainty. However, the use of these nanomaterials should occur with detailed knowledge of delivery, fate and functioning at the target, and finally release from the body. And that's an area where a lot of unanswered questions remain. | |
| In particular, the question of what happens if (and that still often is a big if) the drug-containing nanoparticles reach their intended target is a crucial one: How do the drug molecules get released from the delivery vehicle? In other words, how does the 'envelope' get opened? What is the fate of the nanoparticles (drugs as well as containers) post opening? New work done by scientists in India is contributing to how the nanoscience community is tackling these issues. | |
| Researchers from the Indian Institute of Technology Guwahati present experimental results which suggest that the specificity of release of encapsulated nanoparticles could be achieved with an appropriate combination of encapsulating materials and the choice of an appropriate enzyme that would cleave the encapsulation to release the nanoparticles. | |
| "We have shown that the release of nanoparticles encapsulated in biofriendly starch by specific enzymes can serve as a prototype model for studying the digestion of biofunctionalized nanoparticles and may open newer research avenues where the stabilization and release of nanoparticles could be achieved using well-known therapeutic biomolecules," Dr. Arun Chattopadhyay tells Nanowerk. | |
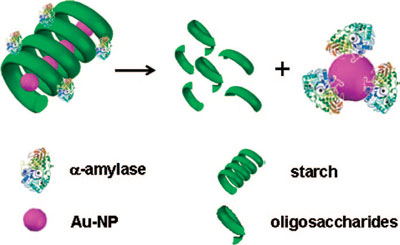 | |
| Schematic representation of the proposed mechanism of gold nanoparticle transfer from the starch-gold nanoparticle composite to the enzyme. The 3D structure of α-amylase is retrieved from Protein Data Base (PDB) entry 1DHK. (Reprinted with permission from American Chemical Society) | |
| Chattopadhyay, a professor in the Department of Chemistry at the Indian Institute of Technology Guwahati, together with his colleagues, has published his findings in the August 20, 2008 online edition of Langmuir ("Probing Au Nanoparticle Uptake by Enzyme Following the Digestion of a Starch-Au-Nanoparticle Composite"). | |
| In this paper, the IIT team reports the results of studies on the enzymatic release of gold nanoparticles encapsulated in starch. | |
| "In particular, we observed that the digestion of a gold nanoparticles-starch composite by α-amylase not only led to the degradation of starch into its lower analogues but also resulted in the release of encapsulated gold nanoparticles and their subsequent uptake by the enzyme" Chattopadhyay explains. "In addition to conventional biochemical and microscopy probes, the surface plasmon resonance (SPR) of gold nanoparticles provided a convenient way of following the reaction and establishing the mechanism. Our observations indicated that the rate of digestion of the starch-gold nanoparticles composite by alpha amylase was similar to that of pure starch and the free thiol groups of the enzyme possibly facilitated the uptake of gold nanoparticles by the enzyme in comparison to other carbohydrate-degrading enzymes such as amyloglucosidase." | |
| These results could be particularly useful for nanoscale drug delivery and imaging studies in vitro. For example, if one wants to screen microorganisms that produce alpha amylase this method would allow a quick and easy way of doing that: the test of the presence of alpha amylase (produced by microorganisms) could be done by the starch-gold nanoparticle composite, which subsequently would release the nanoparticles (catalyzed by the enzyme). Of course, details of the concentrations of the composite etc. would still need to be worked out. | |
| Chattopadhyay gives another example: "If one is interested in screening alpha amylase inhibitors then similar method could be used for that purpose. Alpha amylase inhibitors are known in plants to play important roles in rendering pest resistance attributes to the plants. Hence, there is a tremendous interest in developing transgenic plants bearing such inhibitors. The screening of the inhibitors produced by the plant (or the functional assay of the inhibitors) could be done based on the present method., i.e. measuring the change in localized SPR of gold nanoparticles." | |
| The IIT scientists are currently working on two major areas in the nanomedicine field – fundamental understanding of phenomena related to the development of nanomaterial based diagnostics and therapeutics. | |
| "While conventional diagnostics take either a long time to complete, may involve cumbersome steps, are too expensive to be affordable for a large section of the population, or exhibit low efficiency or sensitivity, the use of nanomaterials could be of great help in overcoming those disadvantages" says Chattopadhyay. "The question is: can one address these downsides systematically, with a reasonably good understanding of the science part of the process? We would like to do that systematically at least in some of the cases." | |
| He mentions that he and his IIT colleagues are also working on the development of nanomaterials-based therapeutics. "For example, we have recently shown that use of silver nanoparticles in conjunction with gene therapy may be a better option for anti-cancer therapy than the use of either of them ("Implications of silver nanoparticle induced cell apoptosis for in vitro gene therapy"). We are currently working on the use of composites rather that use of nanoparticles alone for similar purposes. That way the use of each component (of the composite) would be minimized, while the efficiency of the composite would be better than the isolated components at lower concentrations." | |
| By Michael Berger. Copyright 2008 Nanowerk LLC |
Sunday, August 24, 2008
Buckysomes: Fullerene-Based Nanocarriers for Hydrophobic Molecule Delivery
Department of Internal Medicine, The University of Texas Health Science Center at Houston, 6431 Fannin Street, Houston, Texas 77030
*Address correspondence to Jodie.L.Conyers@uth.tmc.edu.
ABSTRACT

We report the preparation and preliminary in vitro studies of nanocarriers termed “buckysomes,” which are self-assembled, spherical nanostructures composed of the amphiphilic fullerene AF-1. By inducing AF-1 self-assembly at an elevated temperature of 70 °C, dense spherical buckysomes with diameters of 100−200 nm were formed, as observed by electron microscopy and dynamic light scattering. The amphiphilic nature of AF-1 results in the formation of many hydrophobic regions within the buckysomes, making them ideal for embedding hydrophobic molecules to be tested in a drug delivery scheme. After confirming the cellular internalization of buckysomes embedded with the hydrophobic fluorescent dye 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate, we embedded paclitaxel, a highly hydrophobic anticancer drug. The in vitro therapeutic efficacy of the paclitaxel-embedded buckysomes toward suppression of MCF-7 breast cancer cell growth was compared to that of Abraxane, a commercially available, nanoparticle-albumin-bound formulation of paclitaxel. Notably, the paclitaxel-embedded buckysomes demonstrated a similar efficacy to that observed with Abraxane in cell viability studies; these results were confirmed microscopically. Moreover, negative control studies of MCF-7 viability using empty buckysomes demonstrated that the buckysomes were not cytotoxic. The results of our studies suggest that buckysomes prepared from self-assembly of AF-1 at 70 °C are promising nanomaterials for the delivery of hydrophobic molecules.
Background:
Self assembly of amphiphilic C60 fullerene derivatives into nanoscale supramolecular structures
Friday, August 8, 2008
NanoVector virus may solve nanoparticle drug delivery problems
RALEIGH, NC—North Carolina State University professor Stefan Franzen first learned about a distinctive plant virus when he met his colleague Steven Lommel over a beer in Poland several years ago. He did not immediately realize that the meeting would lead to a new approach to nanoparticle drug delivery.
“We got a little bit lucky there,” says Franzen, who holds a PhD in biophysical and biological chemistry. “Serendipity is part of science too.”
“I had known Steve for a long time,” he says. “When I first learned of the properties of the plant virus he was studying, I didn’t get it. I was staring at it for a year without seeing why it is so advantageous. Then, it began to sink in.”
Franzen and his company, NanoVector, had been “playing with nanoparticles for a while,” in particular gold nanoparticles, for use as a targeted drug delivery mechanism. They presented difficult problems that did not show signs of being solved.
Problems with metal nanoparticles
At first Franzen thought Lommel’s plant virus might work with his gold nanoparticles, and even published some work related to the possibility. But eventually, he decided the gold nanoparticles just had too many disadvantages that “All these people who want to promote metal nanoparticles have to solve,” he says.
Lommel, a Ph.D., is a professor of plant pathology, professor of genetics, and Associate Vice-Chancellor for Research at NCSU. His primary research program is in the areas of plant virology, plant viral pathogenesis and plant genomics. He has been working with plant viruses since 1978.
The Eureka! Moment
Recalling that initial meeting in Poland six or seven years ago, Lommel says, “Stefan and I knew each other from NCSU, but didn’t know each other’s research. We shared a hotel and talked a lot. I told him we had just learned to open and close my virus and that it has a hollow cavity in it.”
That is what eventually led to the “Eureka!” moment, he says. Together, Franzen, Lommel, and Bruce Oberhardt, who had been with the company from its inception, revived the inert NanoVector.
“Since then, we have been developing it,” says Lommel of the "plant nanoparticle."
“Its natural properties give it a real advantage," he says. "It can open and close without falling apart. It has a lot of flexibility and potential.”
He points out that NanoVector is not making a drug, but rather a “drug formulation.” It will allow the company to package many current cancer drugs that are not targeted to cancer cells. Targeting drugs is the whole basic idea behind nanoparticle delivery.
Untargeted drugs that affect your healthy cells as well as cancer cells are why your hair falls out during chemotherapy.
To target, “You want to sequester the drug,” says Franzen. “You want it to go into something. You can do some clever things with polymers, but this is more elegant. It’s easier to control and has the advantage of thousands of years of evolution.”
Franzen and Lommel have had several “epiphanies” over the years since sharing beers in Poland. Recently, they realized the plant virus has a natural loading and unloading mechanism built into it. “It’s an exquisite calcium sensor,” Franzen says.
It has a lot of “up potential
Although it may take additional months of research, that finding means they can control the loading and unloading of the medicine in the virus via calcium.
NanoVector is initially targeting cancer therapies, but Franzen says that once the company overcomes regulatory issues, the system could be used to administer pain drugs or any other targeted medicines. “It has a lot of up potential,” he says.
Lommel notes that the company has received grant money and funding from private sources. “We’re doing a dance with some angel investors now,” he says.
The company brought in serial entrepreneur Albert Bender, also a PhD, who was founder and CEO of four venture-backed startups, as CEO.
“We have a division of labor here,” says Lommel. “I’m not doing the business side after spending the last 30 years studying plant viruses.”
MONDAY: Part Two: The business side of NanoVector.
Source
NanoVector
http://www.nanovector.it/
http://www.nanovector.it/brochure_nanovector.pdf
J Am Chem Soc. 2007 Aug 18; : 17705477 (P,S,E,B,D)
Encapsidation of Nanoparticles by Red Clover Necrotic Mosaic Virus.
Lina Loo, Richard Guenther, Steven Lommel, Stefan Franzen
Icosahedral virus capsids demonstrate a high degree of selectivity in packaging cognate nucleic acid genome components during virion assembly. The 36 nm icosahedral plant virus Red clover necrotic mosaic virus (RCNMV) packages its two genomic ssRNAs via a specific capsid protein (CP) genomic RNA interaction. A 20-nucleotide hairpin structure within the genomic RNA-2 hybridizes with RNA-1 to form a bimolecular complex, which is the origin of assembly (OAS) in RCNMV that selectively recruits and orients CP subunits initiating virion assembly. In this Article, an oligonucleotide mimic of the OAS sequence was attached to Au, CoFe2O4, and CdSe nanoparticles ranging from 3 to 15 nm, followed by addition of RNA-1 to form a synthetic OAS to direct the virion-like assembly by RCNMV CP. Dynamic light scattering (DLS) and transmission electron microscopy (TEM) measurements were consistent with the formation of virus-like particles (VLPs) comparable in size to native RCNMV. Attempts to encapsidate nanoparticles with diameters larger than 17 nm did not result in well-formed viral capsids. These results are consistent with the presence of a 17 nm cavity in native RCNMV. Covalent linkage of the OAS to nanoparticles directs RNA-dependent encapsidation and demonstrates that foreign cargo can be packaged into RCNMV virions. The flexibility of the RCNMV CP to encapsidate different materials, as long as it is within encapsidation constraint, is a critical factor to be considered as a drug delivery and diagnostic vehicle in biomedical applications.
Source
Viral cargo delivery
14 January 2008US chemists have used a virus capsule to package and release molecules, which could lead to targeted delivery of therapeutic compounds.
Stefan Franzen and his colleagues at North Carolina State University in Raleigh used the red clover necrotic mosaic virus as a vehicle for dye molecules that can be loaded and unloaded on demand.

|
To explore its versatility for nano-packaging and delivery, Franzen first worked on capturing dye molecules into the capsid. As divalent ions are integral to the virus structure, Ca2+ and Mg2+ depletion in the solution induces significant conformational changes. This leads to surface pores forming, allowing dye molecules to infuse into the interior cavity. Restoring the ion balance closes the pores, trapping the dye inside the virus. When Franzen lowered the ion concentration, the pores reopened and the dye molecules were released.
Franzen's final aim is to use the capsids for intracellular drug delivery - the next stage is to study their ability to package and deliver cargo into a target cell, he explained. The idea is that loaded viruses should be triggered to open their surface pores and release their package inside a cell where the divalent ion concentrations are low. This concept is 'advantageous because the virus capsid will be able to act as container to protect a cargo until it reaches the targeted cell to be released', explained Franzen.
Michael Spencelayh
[SNIP]
Xiao Xiao, a professor of gene therapy at the University of North Carolina School of Pharmacy, says NanoVector's technology is promising because plant viruses will not infect humans and because human cells do not have a defense mechanism against a plant virus.
The drawback, Xiao says, is that once the plant virus enters a cell, the body will remember and develop a defense system within several weeks. If extended treatment is needed, he says, the body would start to block the virus.
"There's no easy way around that problem," Xiao says.
Bender, CEO of NanoVector, admits that resistance could be a problem. He says the company will research the body's immune response to plant viruses in the coming months.Source
Sunday, April 27, 2008
Rational design of amphiphilic polymers to make carbon nanotubes water-dispersible, anti-biofouling, and functionalizable
Free access
Communication
Article citation: Sangjin Park, Chem. Commun., 2008, DOI: 10.1039/b802057d
Rational design of amphiphilic polymers to make carbon nanotubes water-dispersible, anti-biofouling, and functionalizable
Sangjin Park, Hae-Sik Yang, Dongkyu Kim, Kyungmin Jo and Sangyong Jon
We report rational design of amphiphilic polymers capable of making carbon nanotubes (CNTs) highly water dispersible and resistant to biofouling; such CNTs can be conjugated with bioactive molecules so as to be potential drug delivery vehicles.

